States of Matter and Kinetic Theory
Understand how particles behave in solids, liquids, and gases using kinetic theory
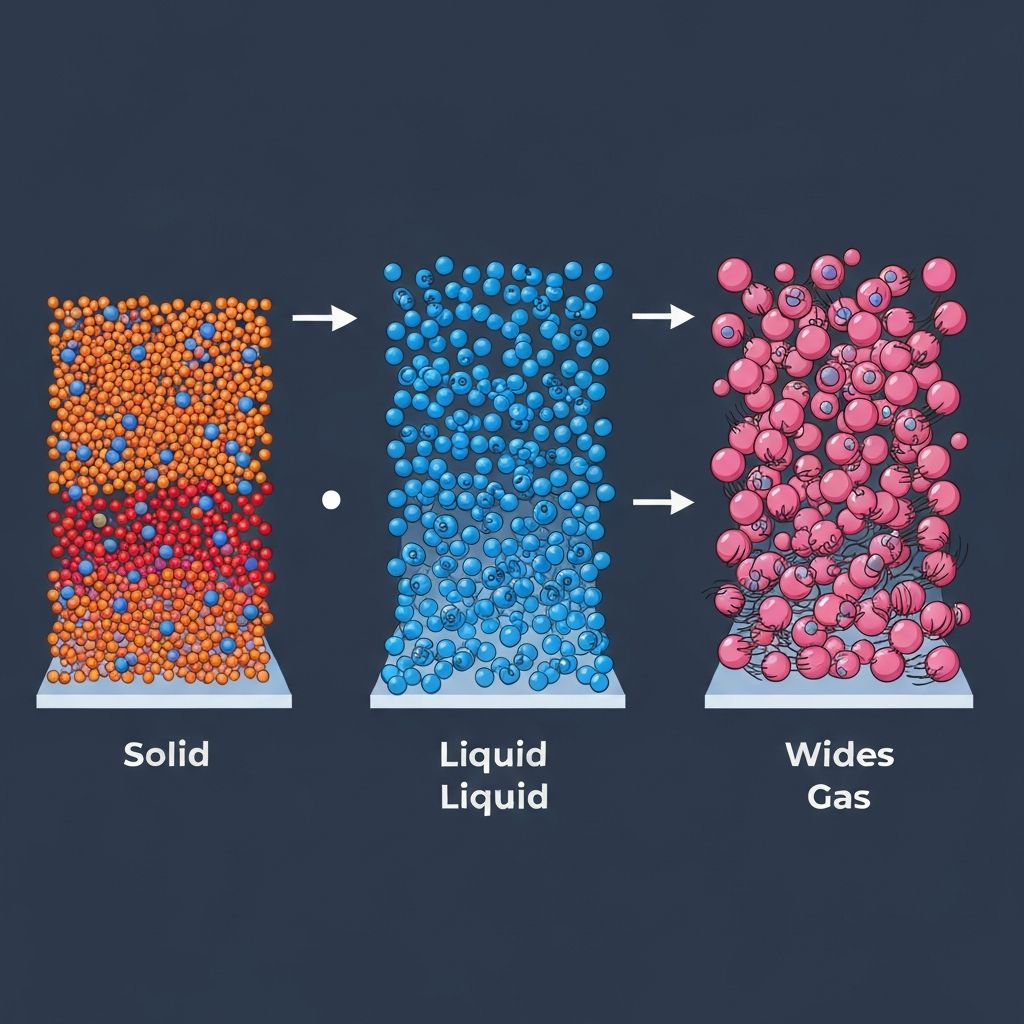
The Three States
Solid, Liquid, Gas
All matter exists in one of three states: solid, liquid, or gas. The kinetic theory explains these states based on how particles are arranged and how they move. In all states, particles are constantly moving, but the amount of movement depends on the energy they possess.
In solids, particles are packed tightly in fixed, regular positions. They can only vibrate about these fixed points because strong intermolecular forces hold them together. This gives solids a fixed shape and volume. Examples include ice, iron, and diamond.
In liquids, particles are still close together but can slide past each other. The intermolecular forces are weaker than in solids, allowing movement. Liquids have a fixed volume but take the shape of their container. Water, oil, and mercury are common examples.
In gases, particles are far apart and move rapidly in random directions. Intermolecular forces are very weak, so particles spread out to fill any container. Gases have no fixed shape or volume. Air, steam, and carbon dioxide behave this way.
Key Exam Point
Temperature affects particle movement. When heated, particles gain kinetic energy and move faster. This is why heating a solid can cause it to melt (become liquid) and eventually boil (become gas). The reverse happens when cooling.
Melting occurs when a solid gains enough energy to break some intermolecular bonds, allowing particles to move more freely. The melting point of water is 0°C. Freezing is the reverse—liquid particles lose energy and form a rigid structure.
Evaporation happens when particles at the liquid surface gain enough energy to escape as gas. This occurs at any temperature. Boiling occurs throughout the liquid at a specific temperature (100°C for water). Condensation is when gas particles lose energy and become liquid.
Sublimation is the direct change from solid to gas without passing through the liquid state. Dry ice (solid CO₂) sublimates at room temperature. The reverse process is called deposition.
Density is mass divided by volume (g/cm³). Solids are typically the most dense because particles are packed closely. Gases are least dense because particles are spread far apart. Water is unusual—ice is less dense than liquid water because of its crystalline structure, which is why ice floats.
Gas pressure is caused by particles colliding with the walls of a container. Increasing temperature makes particles move faster, causing more frequent and harder collisions, which increases pressure. Decreasing volume also increases pressure because particles collide with walls more often.
Question:
Using kinetic theory, explain why a solid is generally denser than the same substance as a liquid.
Answer:
In a solid, particles are arranged in a regular, tightly-packed structure with strong intermolecular forces holding them in fixed positions. They can only vibrate, not move around.
In a liquid, particles have more energy and weaker forces between them. This allows them to move past each other, creating small gaps between particles.
Because the same mass of substance occupies a smaller volume when particles are more tightly packed (solid) compared to when there are gaps (liquid), the solid has a higher density (density = mass ÷ volume).